Mole mass problems worksheet answers – Embark on a comprehensive exploration of mole mass problems with our meticulously crafted worksheet answers. Dive into the intricacies of mole mass calculations, unraveling the complexities with clarity and precision. Prepare to elevate your understanding and conquer any mole mass challenge that comes your way.
This worksheet delves into the fundamental concept of mole mass, equipping you with a solid foundation. Through a series of carefully designed problems, you will master the art of calculating mole mass, empowering you to solve even the most intricate chemistry problems with confidence.
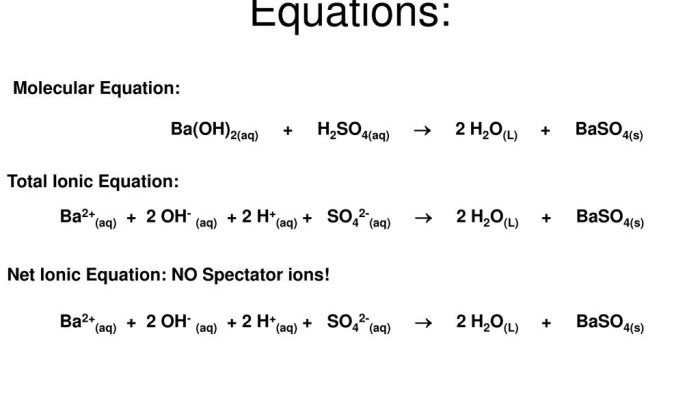
1. Mole Mass Problems Worksheet: Mole Mass Problems Worksheet Answers

Mole mass, a fundamental concept in chemistry, represents the mass of one mole of a substance. It plays a crucial role in stoichiometric calculations, enabling the determination of the quantities of reactants and products involved in chemical reactions. This worksheet is designed to provide a comprehensive understanding of mole mass and its applications in solving various chemistry problems.
1.1 Concept of Mole Mass, Mole mass problems worksheet answers
Mole mass is the mass of one mole of a substance, expressed in grams per mole (g/mol). A mole is defined as the amount of substance that contains exactly 6.022 x 10 23elementary entities, which can be atoms, molecules, ions, or electrons.
The mole mass of an element is numerically equal to its atomic mass, while the mole mass of a compound is the sum of the atomic masses of its constituent atoms.
1.2 Calculating Mole Mass
Calculating the mole mass of a substance involves the following steps:
- Identify the chemical formula of the substance.
- Determine the atomic mass of each element in the formula using the periodic table.
- Multiply the atomic mass of each element by the number of atoms of that element in the formula.
- Add up the masses obtained in step 3 to get the mole mass of the substance.
1.3 Methods for Solving Mole Mass Problems
There are several methods for solving mole mass problems, including:
- Direct calculation using the formula: mole mass = (atomic mass of element 1 x number of atoms of element 1) + (atomic mass of element 2 x number of atoms of element 2) + …
- Using a mole mass table, which provides the mole masses of common elements and compounds.
- Converting between mass and moles using the mole mass as a conversion factor.
Common Queries
What is mole mass?
Mole mass is the mass of one mole of a substance, expressed in grams per mole (g/mol).
How do I calculate mole mass?
To calculate mole mass, add up the atomic masses of all the atoms in the chemical formula of the substance.
What are some common mistakes students make when solving mole mass problems?
Common mistakes include using the wrong atomic masses, forgetting to convert units, and misinterpreting the problem.